Across Europe, a novel method of birth control is emerging. It moves away from daily pills, implants, and uncomfortable fittings. It employs micro-engineering to toggle fertility on and off, while keeping the body’s hormones unchanged.
What the device is and how it works
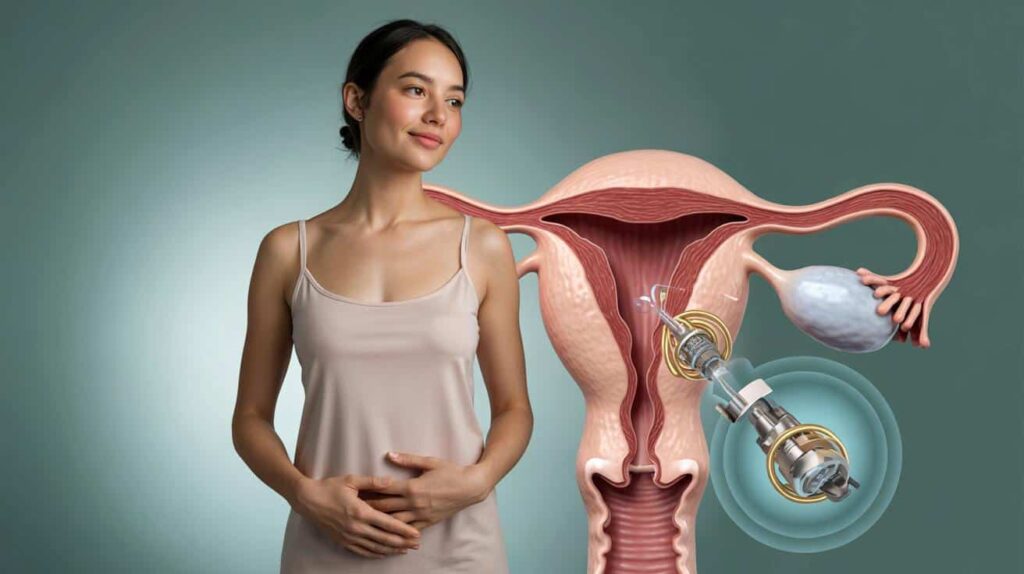
The idea is straightforward to explain but challenging to engineer. Ultra-thin valves are positioned within the fallopian tubes. When the valves close, sperm cannot encounter the egg. When they open, fertility is restored to its original state. No synthetic hormones enter the bloodstream.
Placement will commence in clinics, utilizing a microcatheter guided by a specialist. The company behind the system, Choice, states that the ultimate aim is autonomy. The vision: a device that can be controlled without the need for an operating room, with settings tailored to your plans and comfort level.
Open for natural cycles. Closed for contraception. No hormones. The change occurs locally within the tubes, not throughout the entire system.
The implant combines several components in a small piece of hardware: a valve, a stent to secure it, an antenna, a control circuit, and a power source. A custom micromotor operates the valve with precise movements. Control signals are transmitted to the device wirelessly.
Why a hormone-free route matters
Millions of women tolerate hormones effectively. However, many do not. Copper IUDs avoid hormones, but they may increase menstrual flow and cramping. Some implants raise concerns about migration or difficult removal. Pills carry vascular risks for certain individuals and can influence mood and libido.
A local, reversible, non-hormonal option expands the available choices. It also provides an opportunity for individuals who cannot use hormones, including those with a history of hormone-sensitive cancers such as specific breast cancers.
- No daily pill to remember, no systemic exposure.
- Nothing in the uterus, which can be important for comfort and bleeding patterns.
- On-off control aligned with life events: no surgery required for routine adjustments.
- Potential use after pregnancy or treatment, once a clinician confirms eligibility.
Inside the micromotor
The motor is developed by SilMach, a French expert in hybrid MEMS (micro-electro-mechanical systems). It measures approximately 10 mm by 1 mm, and about 0.1 mm thick. That is thinner than a fingernail and slimmer than a pencil lead. It utilizes electrostatic comb-drive structures to generate motion with tiny, repeated steps. This method produces minimal heat, conserves energy, and provides predictable force.
Smaller than a pencil lead, the module integrates the motor, power source, antenna, controller, valve, and a securing stent into a single implant.
Power delivery was the challenging aspect. Initial tests using focused acoustic energy did not provide sufficient usable energy to reliably move a valve. Choice collaborated with Eindhoven University of Technology to model new transfer methods that can deliver several times the energy typically available to devices of this size, while ensuring tissue safety remains a priority. This work laid the groundwork for SilMach’s custom drive to manage valve movement with consistent accuracy.
The project, partners and timeline
Choice is leading the contraceptive system. SilMach is responsible for building the motor and the hybrid microsystem. The Eurostars program, supported by the European Union, allocated €450,000 for 2022–2025 to advance the design to a working prototype. The teams aim to initiate clinical trials in the Netherlands in the fourth quarter of 2026 after final integration and bench testing. The commercial launch is projected to be several years away, currently aimed for around 2032.
Funded under Eurostars, progressing to first-in-human studies in 2026, with market entry expected in the next decade, pending results and approvals.
Regulatory path and what to watch
The device will need to comply with modern medical device regulations in Europe under the MDR. This entails design controls, cybersecurity for the wireless functions, human-factors testing, and evidence regarding long-term safety. A U.S. pathway would likely follow a de novo or PMA approach. Both markets will require comprehensive data on failure modes, removal, and fertility recovery after years of use.
Key questions remain. How many open/close cycles can the valve achieve over its lifetime? What is the confirmed risk of ectopic pregnancy if a valve fails to close or partially closes? How will clinicians verify valve status at home and in the clinic? MRI compatibility, command encryption, and safeguards against unintended activation will also be scrutinized.
How it compares with existing options
| Method | Hormones | User control | Typical insertion | Noted considerations |
|---|---|---|---|---|
| Fallopian microvalve system (in development) | No | On/off via wireless control, clinic placement initially | Microcatheter into fallopian tubes | Requires imaging and follow-up; long-term data pending |
| Copper IUD | No | On/off requires clinician removal | Inserted through cervix into uterus | Heavier bleeding or cramps for some users |
| Hormonal pill/implant | Yes | Pill requires daily adherence; implant necessitates a procedure | Oral or subdermal placement | Systemic effects; contraindications for some conditions |
What this could change for daily life
Imagine less planning around cycles. No trips to the pharmacy. No uncertainty about whether a missed pill is significant. For couples, the ability to pause contraception for a month and then resume could align better with real-life situations. For clinicians, the focus shifts from renewing prescriptions to checking devices and providing counseling. For payers, costs transition from recurring medications to a one-time implant plus monitoring.
The device does not offer protection against sexually transmitted infections. Condoms and testing will continue to play their role. Dual protection will still be advisable for many individuals.
Context from past attempts
Approaches involving the fallopian tubes are not new. Hysteroscopic sterilization devices once aimed for permanent blockage through tissue growth. They provided insights on placement, pain, migration, and the importance of thorough long-term follow-up. This new system takes a different approach: mechanical valves, reversible action, and electronic control. Even with this distinction, vigilance will be essential. Clear protocols for removal and a swift return to baseline function will be as crucial as the initial placement.
Practical questions and useful details
- Verification: Users will require a dependable method to confirm whether the valve is open or closed. Imaging, sensor feedback, or both can assist.
- Fail-safe design: A secure default state minimizes risk. Engineers must define what “safe” means in everyday life and emergencies.
- Security: Commands must be authenticated. Encryption and proximity limits decrease the likelihood of interference.
- Durability: Materials must withstand corrosion and biofouling within the tubes for many years.
- Access: Training for clinicians and equitable coverage will influence adoption more than technology alone.
If you are picturing the procedure
A microcatheter navigates through the uterus to the tubal opening. The stent gently positions the valve in place. No incisions are made on the abdomen. Recovery should be brief, similar to many diagnostic hysteroscopic procedures. A confirmation test would likely follow to verify placement and initial function before relying on the device for birth control.
Extra notes that broaden the view
Fertility is not binary in reality. Ovulation can vary, sperm can survive for days, and tubes can experience spasms. Any on-off system will require a buffer strategy during transitions. A backup method during the first cycle after a mode change is sensible until the status is confirmed.
Regarding cost and access, a single device that lasts for years may seem expensive initially but could be more economical over time. Health systems often evaluate this with five- or ten-year projections. For patients paying out of pocket, flexible coverage and affordable follow-ups will be significant. Researchers can simulate impact by comparing discontinuation rates, side-effect-related drop-offs, and return-to-fertility intervals across different methods.